CRISPR gene editing has emerged as one of the most revolutionary advancements in gene editing technology, providing promising solutions to persistent genetic disorders. With its ability to precisely target and modify DNA, CRISPR offers hope for treating serious conditions such as sickle cell disease, potentially transforming countless lives. However, the rise of this powerful tool also brings forth significant ethical implications, prompting discussions on the moral responsibilities associated with gene therapy. As society grapples with the medical ethics in gene therapy, the balance between innovation and ethical concerns grows more critical. The intersection of bioethics and gene editing raises profound questions about what it means to be human and the decisions we make regarding our genetic future.
The revolutionary technique known as CRISPR gene editing, a cutting-edge form of genetic manipulation, has the potential to redefine our approach to hereditary diseases. Often described as a molecular scissor for its ability to cut and edit genes with unprecedented accuracy, this technology opens doors to innovative treatments, particularly for ailments like sickle cell anemia. However, as we delve into the potential of this game-changing tool, we must also confront the wider ramifications, including complex ethical considerations that challenge our understanding of medical responsibility. The discussions surrounding bioethics and gene editing reflect a broader societal debate about the extent to which we should intervene in natural processes through medical advancements. As we explore the implications of gene editing, it becomes increasingly important to advocate for a balanced conversation that addresses the ethical landscape alongside the scientific possibilities.
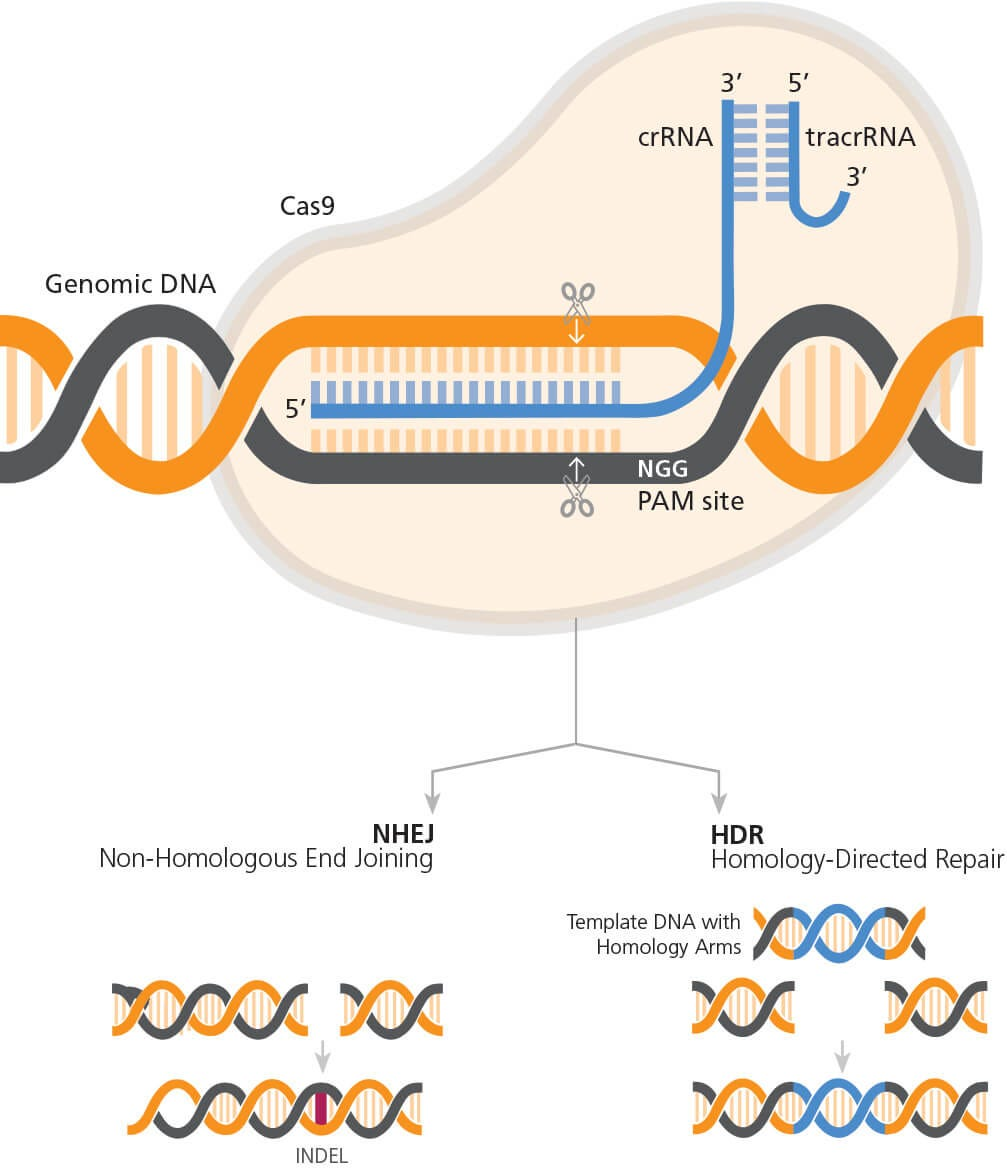
Understanding CRISPR Gene Editing
CRISPR gene editing has emerged as one of the most revolutionary technologies in biotechnology. It allows for precise alterations in the DNA of living organisms, opening doors to potential cures for genetic diseases. By utilizing CRISPR, scientists can target specific genes associated with conditions like sickle cell disease and make modifications that can potentially eradicate these diseases. The technology works by harnessing a natural defense mechanism found in bacteria, adapting it to reprogram DNA sequences, which enhances our capabilities in genetic research and therapy.
However, despite the promising outcomes, CRISPR gene editing raises vital concerns related to its application. The remarkable precision of this technology is counterbalanced by the unpredictability of potential off-target effects, where unintended parts of the genome could be altered. Additionally, as advancements continue to unfold, the conversation surrounding the ethical implications of deploying such powerful tools becomes increasingly important. Questions of accessibility, health equity, and consent emerge, indicating that while CRISPR may have the potential to revolutionize healthcare, it also necessitates stringent oversight and ethical consideration.
Ethical Implications of CRISPR in Medicine
The unfolding narrative surrounding CRISPR gene editing traverses a complex landscape of ethical implications. As Neal Baer discussed during his presentation, the ability to edit genes carries with it a profound responsibility. For instance, the prospect of curing conditions like sickle cell disease unveils a myriad of questions that are not just medical but moral. Should the manipulation of genes extend to non-life-threatening conditions, and who holds the authority to make these weighty decisions? As genetic editing becomes integral to treatment, ethical frameworks must evolve to address such questions.
Moreover, the ethical discourse surrounding CRISPR is numerous, covering the medical, societal, and philosophical dimensions. Bioethicists like Rebecca Brendel emphasize the necessity for reflecting on health justice. The disparity in access to gene editing technologies raises questions about who benefits from such innovations. Will CRISPR deepen existing healthcare divides by favoring those who can afford to pay for gene therapy? Addressing these considerations is crucial to prevent potential inequities that could arise from advanced gene editing.
The Debate Over Sickle Cell Disease Treatment
Sickle cell disease, a painful genetic disorder affecting a significant number of individuals, presents a poignant case study in the potential of CRISPR gene editing. The technology has offered hope for curing this debilitating condition, which often leads to severe health complications, including strokes and chronic pain. Many healthcare professionals argue passionately that the ability to treat and potentially eradicate this disease is a monumental advancement in medical science. With treatments like those made possible through CRISPR, individuals previously facing permanent suffering may now have paths to health and well-being.
Despite this optimism, ethical considerations loom large over the discussion of genetic interventions for sickle cell disease. Critics of widespread genetic modification argue that such approaches should be approached with caution, especially considering the socioeconomic landscape. The exorbitant costs associated with gene therapy raise pertinent questions about the inequity of access to such treatments, highlighting the risk of creating further health disparities. As CRISPR technology continues to evolve, it becomes essential to navigate these challenges responsibly, ensuring that health equity is a foundational pillar in the development and implementation of these groundbreaking therapies.
Navigating Medical Ethics in Gene Therapy
Medical ethics plays a crucial role in the discourse surrounding gene therapy and CRISPR technology, advocating for patient rights and welfare. With the power to modify human genetics comes an obligation to consider the moral consequences of such actions. The discussions by Baer and Brendel at the Harvard event serve as a reminder that one must tread carefully, incorporating ethical accountability into the thrill of scientific discovery. For instance, safeguarding against potential coercion in treatment decisions is essential, especially when the consequences of genetic alterations may profoundly affect individuals and future generations.
Furthermore, the intersection of medical ethics with gene editing prompts conversations about informed consent and the autonomy of patients. Are individuals and families properly informed about the implications of gene editing on their health and future offspring? As gene therapy becomes more commonplace, ethical frameworks must evolve to provide clarity, ensuring that patients are empowered to make informed decisions without undue influence from external pressures, particularly in economically or socially vulnerable populations.
Bioethics and the Future of Gene Editing
Bioethics is increasingly at the forefront of discussions surrounding the future of gene editing, particularly with innovations like CRISPR that challenge traditional notions of medical intervention. This field examines the ethical, legal, and social implications of biotechnological advances, pressing for ethical rigor in the face of rapid development. As scientists explore the boundaries of gene editing, bioethicists are tasked with anticipating potential consequences and advocating for frameworks that prioritize humanity and equity.
One of the critical areas bioethics addresses is the balance between innovation and the precautionary principle. While the potential benefits of gene editing are immense, the unintended consequences of modifying human genomes cannot be overlooked. For example, alterations made with a vision of curing disease might lead to unforeseen interactions within the genetic makeup, which could manifest in new health challenges. Thus, bioethics plays a pivotal role in shaping not just the technology but also how society approaches the profound powers and responsibilities of gene editing.
Health Equity in Gene Editing Technology
Health equity remains a contentious issue as gene editing technologies progress at an unprecedented rate. The disparity in access to advanced treatments like CRISPR gene editing necessitates an urgent examination of the implications for marginalized communities who may be left behind. As seen with treatments for sickle cell disease, the financial burden can be prohibitive, furthering health inequities among different socioeconomic groups. Therefore, it is essential to prioritize inclusivity in the development and distribution of gene editing technologies.
To ensure that all individuals can benefit from gene therapies, stakeholders must engage in discussions about policy-making, funding, and access frameworks that promote equitable healthcare. Initiatives aimed at making CRISPR technology accessible to diverse populations will not only improve overall public health but also foster a sense of fairness and justice in the medical landscape. As gene editing evolves, so too should our commitment to uphold the principles of health equity, ensuring that innovations serve humanity as a whole.
Public Perception and Acceptance of Gene Editing
Public perception of gene editing, particularly CRISPR, is a complex tapestry woven from hope and apprehension. While many view gene editing as a beacon of hope for curing genetic diseases, others express apprehension regarding the ethical implications and potential misuse of such power. As media portrayals often sensationalize the possibilities of genetic manipulation, informed discussions grounded in scientific facts and ethical considerations become critical to fostering public understanding and acceptance.
Engaging communities in dialogue about gene editing can demystify the technology and address common concerns. Educating the public about the science behind CRISPR, alongside its potential benefits and risks, is vital for cultivating trust. Moreover, emphasizing the ethical frameworks guiding research and clinical applications can mitigate fears associated with the unknown. By fostering a transparent conversation about gene editing technology, society can better navigate the intricate balance of innovation and ethical responsibility.
The Role of Regulation in Gene Editing
Regulation plays an integral role in governing gene editing practices to ensure safety, effectiveness, and ethical compliance. As CRISPR technology continues to evolve, regulatory frameworks must adapt to address the unique challenges posed by this powerful tool. Effective oversight not only ensures compliance with existing laws but also helps to prevent unethical practices, such as unregulated gene editing or disregard for informed consent. Collaboration between scientists, ethicists, and regulatory bodies is essential for establishing standards that protect both patients and future generations.
Furthermore, international collaboration on regulatory measures is crucial as gene editing knows no borders. With advancements occurring globally, a unified approach could mitigate risks associated with disparate legal frameworks, particularly in countries with less stringent regulations. Thus, proactive engagement in international discussions will help establish guidelines that prioritize human rights and ethical concerns across all nations, reinforcing a commitment to responsible research and application in gene editing.
The Future of Gene Therapy: Innovations and Challenges
As the field of gene therapy rapidly advances, innovations through CRISPR and other gene editing technologies hold the potential to reshape the future of medicine. Researchers are exploring ways to enhance the precision and safety of gene modifications, enabling targeted treatments for a myriad of conditions. However, as innovation progresses, so too does the imperative to confront the associated challenges, particularly those rooted in ethics and access. Ensuring that these life-saving techniques reach all corners of society remains a significant hurdle for researchers and healthcare policymakers.
Looking ahead, the future of gene therapy hinges on addressing barriers to accessibility and fostering collaborative dialogues among stakeholders. Innovation will only be fruitful if it is coupled with a commitment to health equity and ethical practices. Engaging in proactive discussions centered on the ethical implications and societal impacts of gene editing will set the stage for a future where technological advancements serve to benefit all individuals, transcending social, economic, and geographical divides.
Frequently Asked Questions
What are the implications of CRISPR gene editing for treating sickle cell disease?
CRISPR gene editing offers promising implications for treating sickle cell disease by allowing scientists to modify somatic cells to correct the genetic mutations causing the disease. This could potentially cure individuals suffering from sickle cell anemia and drastically improve their quality of life. However, the use of CRISPR in this context raises significant ethical implications, particularly concerning access and health equity, as such treatments can be expensive and may not be available to all who need them.
What are the ethical implications of using CRISPR gene editing on human embryos?
The ethical implications of using CRISPR gene editing on human embryos are profound. This technology could permit alterations to germline genes, possibly eradicating hereditary diseases before birth. However, it leads to debates about ‘designer babies,’ parental control over child traits, and the long-term impacts on human diversity and identity. Furthermore, it raises questions regarding the oversight of such practices and the potential for misuse without appropriate regulatory frameworks.
How does gene editing technology like CRISPR affect discussions on medical ethics in gene therapy?
Gene editing technology like CRISPR significantly enhances discussions on medical ethics in gene therapy by introducing complex dilemmas related to consent, potential for unintended consequences, and the moral permissibility of editing genes that contribute to non-life-threatening conditions. It challenges our understanding of what constitutes a ‘treatment’ versus a choice to enhance human capabilities, necessitating robust bioethical frameworks to guide responsible use in medical contexts.
What role do bioethics play in harnessing CRISPR gene editing for disease treatment?
Bioethics play a crucial role in harnessing CRISPR gene editing for disease treatment by ensuring that developments in science align with moral values and societal norms. As we explore cures for diseases like sickle cell, bioethics provide guiding principles to address issues such as equity, consent, and the potential for long-lasting societal impacts, ensuring that innovation does not outpace ethical considerations.
Can CRISPR gene editing lead to equitable health outcomes in treating genetic diseases?
CRISPR gene editing has the potential to lead to equitable health outcomes in treating genetic diseases, but significant barriers remain. Access to this technology can be highly variable based on socioeconomic status, geography, and healthcare infrastructure, potentially exacerbating existing health disparities. Ensuring equitable access requires proactive policy interventions and ethical commitments to health justice in the deployment of CRISPR therapies.
What are the risks associated with unintended consequences of CRISPR gene editing?
The risks associated with unintended consequences of CRISPR gene editing are considerable. While gene editing can correct specific mutations, it may inadvertently disrupt other essential genes or create off-target effects that could lead to new health issues. These complexities highlight the necessity of thorough research and testing before clinical applications, as well as a careful evaluation of the long-term effects on genetic integrity and health.
How does CRISPR gene editing intersect with the concept of genetic determinism?
CRISPR gene editing intersects with the concept of genetic determinism by raising questions about the extent to which genes control physical and psychological traits. The ability to modify genes invites discussions about whether our genetic makeup determines our identity or capabilities, and to what extent we should intervene in our genetic legacy. This relationship prompts a reevaluation of cultural and ethical frameworks regarding human variation and diversity.
| Key Points |
|---|
| CRISPR technology allows for editing of both somatic and germline genes, offering potential cures for genetic diseases like sickle cell anemia. |
| The ethical implications of using CRISPR for conditions compatible with life, such as Down syndrome, raise significant concerns regarding decision-making. |
| The high costs of gene therapy, exemplified by the sickle cell cure priced at $2.2 million, pose questions about accessibility and equity in healthcare. |
| There is a debate on whether parents should decide on genetic modifications for their children, especially regarding non-pathological conditions. |
| Concerns about oversight and regulation of gene editing technologies highlight the risk of unmonitored practices in different countries. |
| Gene editing may have unintended consequences, as genes interact in complex ways over millions of years of evolution. |
Summary
CRISPR gene editing is a revolutionary technology that offers the potential to cure genetic diseases but brings forth a myriad of ethical dilemmas. As discussions surrounding its use unfold, it is important to carefully weigh the benefits against the implications for society and individual rights. The need for comprehensive oversight and consideration of health equity highlights the profound responsibility that comes with such powerful scientific advancements.