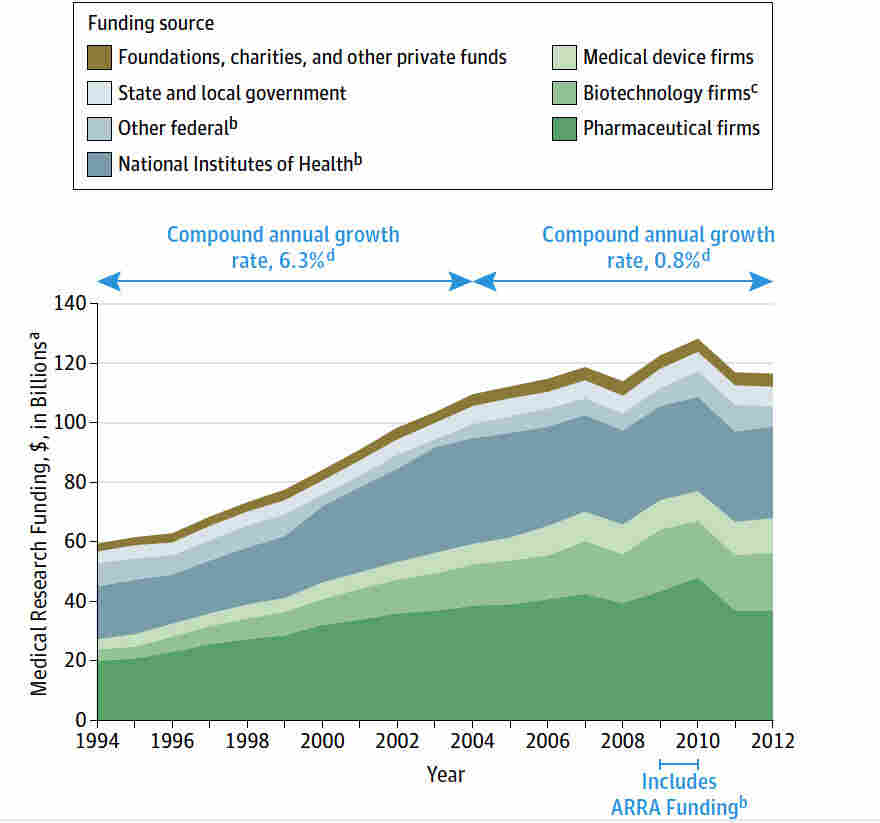
Medical research funding is critical for ensuring the safety and efficacy of clinical trials that contribute to advancing healthcare. Recent funding cuts have jeopardized essential oversight mechanisms, raising concerns about the impact of funding cuts on research efficacy and patient safety in clinical trials. The role of Institutional Review Boards (IRBs) has become increasingly important, as they manage the ethical conduct of research and safeguard the safety of research participants. Without adequate financial resources, the comprehensive review process provided by IRBs could face disruptions, putting vulnerable populations at risk. Institutions like Harvard Medical School, with their robust research frameworks, are at the forefront of addressing these challenges, striving to protect both patients and the integrity of scientific inquiry.
Funding for medical investigation is fundamental to maintaining rigorous standards in healthcare research. When financial support is diminished, it disrupts not just the flow of research but also the essential protections designed to uphold participant well-being and ethical standards. As institutions grapple with economic constraints, the need for effective IRB oversight becomes paramount to ensure that the complexities of patient safety in clinical trials are not compromised. The conversation surrounding research financing has become urgent, especially as it underscores the repercussions of halted projects and the need for sustainable funding solutions. Institutions like Harvard, renowned for their contributions to medical research, play a pivotal role in advocating for patient rights and advancing successful research outcomes.
Understanding the Impact of Funding Cuts on Medical Research
Funding cuts have a profound effect on the scope and quality of medical research. When financial resources are slashed, as seen with the recent halt to over $2 billion in federal research grants at Harvard, projects that aimed to ensure the rights and safety of patients suffer greatly. Research efforts are intertwined with funding; hence, the loss of financial support directly translates into limitations in research capabilities. Researchers are faced with the challenge of continuing their work while managing the adverse effects of these cuts on their studies, which often leads to the postponement of crucial clinical trials and innovative research endeavors.
Additionally, the halt in funding exacerbates existing issues surrounding patient safety in clinical trials. With fewer resources, the oversight necessary to protect participants is compromised. Institutional Review Boards (IRBs) play a crucial role in ensuring that all ethical standards are maintained, but without adequate funding, their operations can become hampered. As a result, the safety of research participants comes under threat, as there may not be enough oversight to monitor risks and comfort levels within clinical trials.
The Role of IRB Oversight in Maintaining Research Ethics
Institutional Review Boards (IRBs) are vital for protecting the rights and welfare of human subjects involved in research. They meticulously review research proposals to safeguard participants from potential harm, while ensuring informed consent is adequately obtained and understood. In light of recent funding cuts, the operations of these IRBs are jeopardized, affecting their ability to fulfill this essential oversight role. The importance of IRBs cannot be understated; their checks and balances ensure ethical considerations are prioritized in the medical research process.
Moreover, without sufficient funding, the education and training programs that prepare IRB members and researchers may diminish, leading to less informed oversight. This is particularly concerning when you consider the historical failures in medical research ethics. Modern IRBs have been developed to prevent such occurrences, but their effectiveness is directly related to sustained funding and support. The cutbacks in funding potentially undermine the foundation of ethical research, compromising the safeguards established to protect research subjects.
Harvard Medical Research and Its Vital Contributions
Harvard Medical School has long been at the forefront of groundbreaking medical research aimed at improving patient outcomes. As a leading institution, it conducts extensive studies that inform treatment practices and medical policy on a global scale. However, recent funding cuts threaten not just Harvard’s capabilities but the overarching landscape of medical innovation. The collaboration among researchers, many of whom are linked through initiatives like the SMART IRB, is essential for tackling complex health challenges effectively.
Harvard’s research funding plays a pivotal role in transformative studies, influencing thousands of patients. The reliance on federal research funding means that any disruption can lead to delays in significant discoveries and clinical advancements. As researchers at Harvard and similar institutions work to develop new therapies, the cuts have long-lasting effects that extend beyond the confines of a single institution, impacting public health research comprehensively.
Patient Safety Considerations in Light of Research Funding Cuts
The safety of research participants is paramount, especially in medical trials where novel interventions are being tested. Funding cuts have significantly impacted this critical aspect, as resources that typically support comprehensive monitoring and oversight are curtailed. When funding is reduced, many clinical studies find it increasingly challenging to adhere to strict protocols that ensure the safety of those involved. This can lead to an increase in adverse events and might even result in attrition from important research studies.
Moreover, when overseeing the safety of participants, IRBs face the challenge of limited financial resources, which constricts their ability to review protocols thoroughly. The potential for increased risks to participants, combined with public skepticism about research fidelity, poses a double-edged sword for clinical studies. Researchers and institutions must strive to maintain ethical standards, but the implications of funding cuts make it significantly more difficult to protect those who volunteer for studies.
Addressing the Challenges of Collaborative Research Amidst Funding Cuts
Collaborative research is essential in advancing medical knowledge, particularly in the era of complex health issues that require unified approaches. The SMART IRB system exemplifies these collaborations, streamlining the review process across multiple sites. However, funding disruptions hinder the ability of researchers to add new collaborators, resulting in significant delays in clinical trials. The more institutions involved in a study, the wider the potential impact; yet, with funding cuts halting the necessary groundwork, many promising projects are stalled.
The interconnectivity of institutions within the Harvard research network allows for innovative solutions to health issues to emerge quickly, but funding limitations significantly compromise this synergy. When federal support is jeopardized, those involved in collaborative studies struggle to maintain cohesion. This can ultimately result in less robust research findings and a slower pace of innovations translated into real-world applications.
Long-Term Consequences of Federal Funding Cuts in Research
The long-term consequences of funding cuts in medical research can be detrimental, not just for specific studies but for the broader scope of healthcare advancements. As noted, halting ongoing projects creates a ripple effect that can delay critical findings essential for patient care improvement. Medical innovations, which often rely on substantial research funding, can stagnate, impacting future healthcare strategies and treatment options available to patients.
Furthermore, the erosion of trust between research institutions and the communities they serve can take years to rebuild, if at all. Public perception is crucial for patient participation in clinical research; funding cuts may foster skepticism or reluctance to participate. To maintain a healthy and effective research environment, sustained investment is essential, ensuring that ethical standards remain in place and that researchers can carry out their essential work without compromise.
The Necessity of Ethical Oversight in Medical Trials
Ethical oversight in medical trials is non-negotiable as it directly correlates with the safety and efficacy of the research being conducted. Fundamental to this oversight is the role of IRBs, which evaluate each study proposal to ensure that ethical guidelines are followed, protecting the rights of participants. The regulatory frameworks that emerge from stringent IRB oversight help to mitigate risks and ensure that research complies with established ethical standards.
However, with recent funding cuts, the support systems that allow IRBs to function effectively are strained. Reducing the operational capabilities of IRBs and associated regulatory bodies leaves gaps in ethical oversight that can lead to increased risks for participants. This calls for immediate attention to ensure that even amidst budgetary constraints, ethical considerations remain a priority in the conduct of medical research.
Future Directions for Medical Research Funding
Moving forward, the landscape of medical research funding will need reevaluation to safeguard against future disruptions. An urgent response to the detrimental impact of funding cuts must include strategic planning that prioritizes continued support for essential research initiatives. Developing legislation and policies that protect federal funding allocations for medical research is crucial in nurturing an environment conducive to innovation and patient safety.
Additionally, increasing awareness and advocacy for robust funding can mobilize public and private sectors to contribute resources. Collaborative efforts among stakeholders—including healthcare professionals, researchers, and policymakers—can jointly address the challenges posed by funding shortages. By establishing sustainable funding sources and demonstrating the wider health impacts of research, the medical community can work towards a future where funding is less uncertain and more robust.
The Importance of Community Engagement in Research Oversight
Community engagement is a critical component of ethical research practices, particularly in obtaining informed consent and fostering trust. By actively involving community members in the decision-making processes of research, institutions can better understand the values and concerns of potential participants. This engagement cannot be overlooked, especially in tumultuous times of funding cuts that could otherwise alienate researchers from the very populations they aim to serve.
Building solid relationships with community stakeholders can enhance transparency and accountability in medical research. When the community views research initiatives as beneficial and trustworthy, it encourages participation and can lead to more robust data collection. This mutual understanding is paramount, especially when external funding is under threat, as it offers a foundation for continued collaboration and enhances public support for essential research activities.
Frequently Asked Questions
How do funding cuts impact the safety of research participants in medical research?
Funding cuts to medical research significantly impact the safety of research participants by disrupting oversight mechanisms such as institutional review boards (IRBs). These boards ensure compliance with ethical standards meant to protect participants’ rights and welfare. For instance, the recent funding freeze at Harvard halted ongoing studies and prevented new sites from joining, posing risks to patient safety in clinical trials.
What is the role of IRB oversight in medical research funding?
IRB oversight plays a crucial role in medical research funding by reviewing research proposals to safeguard participant safety and ethical compliance. With proper funding, IRBs can operate effectively, ensuring that studies adhere to ethical standards and regulations. When funding cuts occur, the ability of IRBs to oversee studies diminishes, potentially compromising patient safety and the integrity of research.
Why is the impact of funding cuts on research a concern for patient safety?
The impact of funding cuts on research is a major concern for patient safety because it leads to delays and disruptions in clinical trials. Without adequate funding, important research initiatives may halt, preventing new therapies from being developed and tested. This not only affects the safety of current participants but also erodes public trust in the research process.
How do health institutions like Harvard ensure patient safety in clinical trials despite funding challenges?
Health institutions like Harvard ensure patient safety in clinical trials through robust IRB oversight and strategic collaborations. Even amid funding challenges, such institutions rely on existing resources and institutional support to continue critical oversight functions. Initiatives like SMART IRB facilitate cooperative research efforts to maintain participant safety across multiple sites.
What historical events highlight the importance of medical research funding for safeguarding participants?
Historical events, such as the Tuskegee Syphilis Study and unethical medical experiments during WWII, underscore the critical importance of medical research funding for safeguarding participants. These events led to the establishment of stringent IRB oversight and regulations aimed at protecting human subjects. Ensuring adequate funding is essential for maintaining these protective measures and preventing a recurrence of past injustices.
In what ways can patients influence the conversation about medical research funding?
Patients can influence the conversation about medical research funding by advocating for transparency and ethical standards in research. Engaging with policymakers, participating in public forums, and sharing personal experiences can heighten awareness of the importance of robust funding for safeguarding patient safety in clinical trials. Their involvement is crucial for driving support for sustained and increased funding.
| Key Points |
|---|
| The Trump administration froze over $2 billion in federal research grants to Harvard, disrupting medical research funding. |
| The freeze affects the SMART IRB, which is vital for overseeing multi-site medical research and protecting patient rights. |
| Institutional Review Boards (IRBs) ensure research compliance with laws and protect the rights and welfare of participants. |
| Recent funding cuts jeopardize ongoing studies, potentially harming patient safety and eroding public trust in research. |
| Historical issues in medical research, like the Tuskegee study, highlight the need for effective oversight provided by IRBs. |
| The work of IRBs is crucial for maintaining ethical standards and enhancing medical research collaboration. |
Summary
Medical research funding is crucial for upholding patient safety and ethical standards in clinical trials. The recent halt in funding, particularly through mechanisms like SMART IRB, threatens the integrity of ongoing research and the protection of participants. As demonstrated, effective oversight by Institutional Review Boards (IRBs) is essential for safeguarding the rights and welfare of those involved in studies. Without the necessary funding, essential research efforts face significant disruptions, which could lead to adverse outcomes for both participants and the larger community that relies on medical advancements.