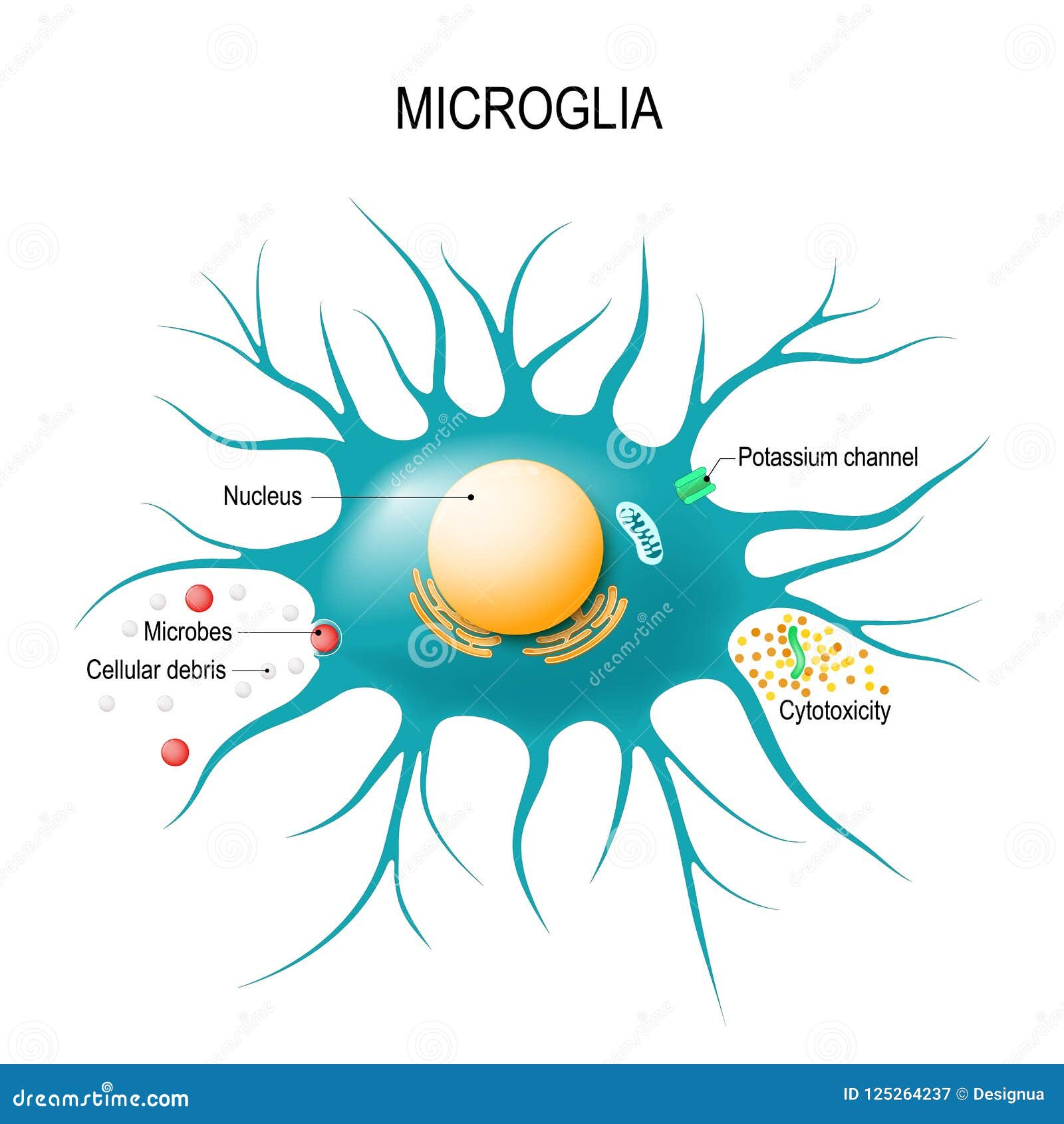
Microglial cells are a critical component of the brain’s immune system, playing an essential role in maintaining neural health and function. These specialized immune cells tirelessly patrol the brain, effectively clearing away dead or damaged neurons and engaging in synaptic pruning to enhance communication between neurons. However, recent findings from Beth Stevens’ research have revealed that dysfunction in microglial activity can exacerbate neurodegenerative diseases like Alzheimer’s disease. In her groundbreaking work, Stevens highlights how abnormal synaptic pruning by microglia may contribute to the progression of conditions such as Alzheimer’s and Huntington’s disease. This transformative research not only deepens our understanding of brain pathology but also paves the way for developing innovative biomarkers and therapies for millions affected by neurodegenerative disorders.
Glial cells, specifically the resident immune cells in the brain known as microglia, serve as crucial defenders within the central nervous system. These cells are not only vital for clearing cellular debris but also play a pivotal role in shaping neuronal connections during brain development through a process known as synaptic pruning. The pioneering investigations led by scientists like Beth Stevens have illuminated the link between microglial dysfunction and various neurodegenerative conditions, including Alzheimer’s and Huntington’s diseases. By analyzing how these cells interact with neurons, researchers are unlocking new insights that may lead to therapeutic breakthroughs for these debilitating disorders. Understanding the dual role of microglia in both health and disease is essential for advancing our strategies against neurodegeneration.
Understanding Microglial Cells in Alzheimer’s Research
Microglial cells play a crucial role in the brain’s immune system, constantly surveying their environment for signs of disease or damage. In the context of Alzheimer’s disease, these cells are responsible for synaptic pruning—removing excess synapses to maintain a healthy neural network. However, recent findings suggest that in conditions like Alzheimer’s, microglia may begin to prune synapses excessively or incorrectly. This abnormal behavior can lead to cognitive decline, making the study of microglial cells essential for understanding how Alzheimer’s and other neurodegenerative diseases progress.
Research conducted by Beth Stevens has pivoted our understanding of microglial function, emphasizing their dual role in brain health and disease. The Stevens Lab has discovered that when microglia malfunction, they can contribute to neurodegenerative processes not just in Alzheimer’s disease, but also in conditions like Huntington’s disease. By exploring these mechanisms, Stevens and her team hope to identify new biomarkers and therapeutic targets that could potentially alter the course of these devastating conditions, underscoring the importance of microglial research in transforming Alzheimer’s treatments.
Beth Stevens and the Evolution of Alzheimer’s Understanding
Beth Stevens, a prominent figure in Alzheimer’s research, has significantly influenced how we comprehend the interactions between microglial cells and neurodegenerative diseases. Through meticulous research, she has illustrated how microglia’s synaptic pruning activities are crucial during normal brain development. However, Stevens also warns of the potential for these processes to become dysregulated, leading to synaptic loss in conditions like Alzheimer’s. Her groundbreaking studies have revealed a connection between abnormal microglial function and the pathology of Alzheimer’s disease, encouraging a reevaluation of how we approach these neurodegenerative conditions.
Moreover, Stevens’ emphasis on foundational research showcases the unpredictable nature of scientific discovery. Her journey into understanding the visual systems in mice, while seemingly unrelated to human disease, paved the way for insights into the pathophysiology of Alzheimer’s. The combination of curiosity-driven research and funding from sources like the National Institutes of Health has been instrumental in uncovering the complexities of the brain’s immune response, pointing to a broader narrative where basic science can lead to meaningful advancements in clinical applications for Alzheimer’s and other neurodegenerative diseases.
The Role of Synaptic Pruning in Neurodegeneration
Synaptic pruning is a vital process mediated by microglial cells that helps sculpt the connections in the brain. In healthy brains, this process is critical for refining neural circuits during development and supporting cognitive function. However, in Alzheimer’s and other neurodegenerative diseases, synaptic pruning can become excessive, leading to significant synaptic loss. Understanding how microglia contribute to synaptic pruning is essential for deciphering the complex biology of neurodegeneration and developing targeted therapies for diseases like Alzheimer’s.
Beth Stevens’ research has shown that abnormal synaptic pruning is associated with the progression of Alzheimer’s disease. Her investigations into the mechanisms behind this dysregulation provide crucial insights into how the immune system interacts with neural tissues. By identifying the pathways involved in microglial activity, researchers are hopeful that this knowledge will lead to more effective strategies for halting or reversing synaptic loss, thereby offering new hope to the millions affected by neurodegenerative diseases today.
Innovative Approaches in Alzheimer’s Biomarkers and Treatments
In the quest to understand Alzheimer’s disease, identifying reliable biomarkers has become a cornerstone of research. Beth Stevens’ lab is leading the charge in exploring the connection between microglial activity and potential biomarkers that signal disease progression. As abnormal synaptic pruning is linked to neurodegeneration, it presents a promising target for developing diagnostic tools. Additional research in this area could lead to predictive markers that allow for earlier detection and intervention, ultimately aiming to slow the disease’s progress.
Moreover, the pursuit of new therapeutic approaches is critical in line with these biomarker developments. By understanding the role of microglial cells in Alzheimer’s disease, scientists can create therapies that not only modulate microglial activity but also promote healthy synaptic function. The integration of Stevens’ findings into clinical strategies marks an exciting advancement in the fight against Alzheimer’s, with potential to greatly improve the quality of life for millions of Americans living with this neurodegenerative disease.
The Neuroscience Behind Neurodegenerative Diseases
Neurodegenerative diseases such as Alzheimer’s and Huntington’s provoke intense research interest due to their profound impact on individuals and society. Understanding the underlying neuroscience is paramount for devising impactful treatments. This involves studying neuronal loss, synaptic dysfunction, and the immune response mediated by microglial cells. As highlighted by Beth Stevens, the intersection of these processes reveals critical insights into how brain health is maintained and compromised in neurodegenerative conditions.
Given the complex nature of these diseases, a multidisciplinary approach combining molecular biology, neuroimmunology, and clinical research is essential. Stevens’ emphasis on comprehensive understanding facilitates collaborations that bridge insights from various fields, enhancing the efficacy of treatment strategies aimed at neurodegenerative diseases. Exploring these avenues not only leads to advancements in Alzheimer’s disease but also sets the stage for understanding other related neurodegenerative conditions, thereby driving forward the landscape of neurological research.
Impact of Federal Funding on Alzheimer’s Research
Federal funding has been vital in propelling research forward in the field of Alzheimer’s disease. Through agencies like the National Institutes of Health, scientists like Beth Stevens have been able to secure the resources necessary to pursue groundbreaking investigations into microglial function and neurodegeneration. This support has paved the way for significant discoveries, particularly in identifying the roles that the brain’s immune cells play in conditions such as Alzheimer’s and beyond.
Moreover, the relationship between foundational research and tangible outcomes cannot be overstated. Researchers are often tasked with addressing fundamental questions that may not initially appear relevant to clinical practice. However, Stevens’ research illustrates how exploration in basic science has far-reaching implications, leading to innovations that could ultimately transform clinical approaches to Alzheimer’s disease. As advocacy for continued support for federal research funding grows, so too does the potential for breakthroughs in treatment and understanding of neurodegenerative diseases.
The Future of Alzheimer’s Therapies and Research
Looking ahead, the future of Alzheimer’s therapies lies in innovative research that integrates findings from recent advances in understanding microglial biology. With scientists like Beth Stevens at the forefront, there is hope that new approaches will emerge, focusing on immune response modulation and synaptic preservation. As technology and research methodologies evolve, the potential for identifying novel therapeutic targets increases, offering hope not just for Alzheimer’s treatment but also for the broader category of neurodegenerative diseases.
Furthermore, the collaborative nature of modern neuroscience research is leading to a more integrated understanding of Alzheimer’s disease. By pooling resources and knowledge across institutions, researchers can delve deeper into the mechanisms at play within the brain’s immune system. This collaborative effort is crucial for discovering effective interventions aimed at reversing the detrimental effects of synaptic pruning and ensuring a more resilient neural environment, ultimately improving outcomes for those affected by Alzheimer’s disease.
The Importance of Curiosity-Driven Research in Neuroscience
Curiosity-driven research is a fundamental pillar of scientific discovery, particularly in the field of neuroscience. Beth Stevens emphasizes that her journey into understanding the brain’s immune system was propelled by an innate curiosity about microglial cells and their function in neurodevelopment. This type of research is critical as it often leads to unexpected findings that can reshape our understanding of diseases, particularly complex conditions like Alzheimer’s.
By prioritizing exploratory and foundational research, scientists can create a rich environment for innovation. Such an approach encourages researchers to ask bold questions and pursue diverse lines of inquiry, often illuminating connections that more targeted studies may overlook. The insights gained from curiosity-driven research can lead to advancements in understanding the mechanisms behind neurodegenerative diseases and, ultimately, the development of new therapeutic strategies.
Challenges and Opportunities in Alzheimer’s Disease Research
The landscape of Alzheimer’s disease research is filled with both challenges and opportunities. One major challenge is the heterogeneity of the disease, which can manifest differently from one individual to another. Understanding the role of microglial cells in these variations is critical, as Stevens’ research suggests that different individuals may experience different degrees of synaptic pruning. This complexity necessitates personalized approaches to treatment and highlights the need for further investigation into the mechanisms driving these variances.
However, these challenges also provide a wealth of opportunities for researchers to innovate. By studying the interplay between microglial dysfunction and synaptic loss, scientists can develop more targeted therapies that could improve patient outcomes. Furthermore, collaboration across various scientific disciplines can forge new paths towards understanding Alzheimer’s disease, paving the way for breakthroughs in clinical practice that could transform the future of care for individuals facing this daunting neurodegenerative challenge.
Frequently Asked Questions
What are microglial cells and their role in the brain’s immune system?
Microglial cells are specialized immune cells located in the brain and spinal cord. They play a crucial role in the brain’s immune system by monitoring for signs of injury or illness and clearing away dead or damaged neurons. Their functions also include synaptic pruning, which is the process of eliminating unnecessary synapses to improve neural efficiency.
How do microglial cells contribute to neurodegenerative diseases like Alzheimer’s?
Microglial cells can significantly impact the progression of neurodegenerative diseases, including Alzheimer’s disease. Abnormal activity in these cells, particularly in their synaptic pruning processes, has been linked to the deterioration of neuronal connections that characterize Alzheimer’s. This disruption can lead to increased inflammation and further damage to brain tissue.
What is the significance of Beth Stevens’ research on microglial cells?
Beth Stevens’ research has revolutionized our understanding of microglial cells, especially regarding their role in synaptic pruning and their implications in neurodegenerative diseases. Her findings indicate that improper microglial function may contribute to the progression of Alzheimer’s disease, paving the way for new biomarkers and therapeutic strategies to combat these disorders.
How can microglial cell dysfunction affect synaptic pruning?
Microglial cell dysfunction can lead to excessive or insufficient synaptic pruning, which may result in the loss of important neural connections or the formation of improper ones. In conditions such as Alzheimer’s disease, this abnormal pruning can exacerbate neural degeneration and cognitive decline, highlighting the delicate balance microglial cells maintain in brain health.
What are the potential therapeutic implications of understanding microglial cells in Alzheimer’s disease?
Understanding microglial cells offers significant therapeutic potential for Alzheimer’s disease. By targeting the pathways involved in microglial activation and synaptic pruning, researchers like Beth Stevens aim to develop new treatments that could halt or reverse the neurodegenerative processes, ultimately improving quality of life for patients.
What protective roles do microglial cells serve during brain development?
During brain development, microglial cells are essential for synaptic pruning, which helps shape neuronal circuits and promote efficient brain function. By selectively eliminating excess synapses, they ensure that the brain develops healthy and functional connections, which are vital for overall cognitive abilities.
Can studying microglial cells help in understanding other neurodegenerative diseases besides Alzheimer’s?
Yes, studying microglial cells not only enhances our understanding of Alzheimer’s disease but also informs research on other neurodegenerative diseases such as Huntington’s disease. Insights from microglial function may lead to more comprehensive strategies to detect and treat various conditions that involve neuroinflammation and synaptic dysfunction.
| Key Points | Description |
|---|---|
| Microglial Cells Function | Act as the brain’s immune system, clearing dead cells and pruning synapses. |
| Role in Alzheimer’s Disease | Abnormal pruning by microglia contributes to Alzheimer’s and other neurodegenerative diseases. |
| Research Impact | The Stevens Lab’s work has led to new biomarkers and therapies for neurodegenerative diseases. |
| Importance of Foundational Research | Basic science drives advancements in understanding brain functions and diseases. |
Summary
Microglial cells play a crucial role in maintaining brain health by acting as the immune system of the brain. Their ability to clear dead cells and prune synapses is essential, but when this process goes awry, it can contribute to devastating neurological diseases like Alzheimer’s. As research led by Beth Stevens highlights, understanding and manipulating the function of microglial cells could unlock new treatments for millions of Americans affected by these conditions. Stevens’ pioneering work, supported by significant federal funding, underscores the necessity and impact of foundational research in advancing our knowledge of brain health and disease.