Alzheimer’s research is at the forefront of tackling one of the most pressing public health challenges of our time. As millions of people grapple with this debilitating neurodegenerative disease, scientists like Beth Stevens are pioneering innovative paths to uncover the role of microglial cells—the brain’s immune defenders. These cells are crucial in maintaining brain health, as they remove damaged cells and ensure proper synaptic pruning, vital for cognitive function. However, dysfunction in this process could contribute to Alzheimer’s, highlighting the necessity for effective Alzheimer’s treatments. By advancing our understanding of these mechanisms, Stevens’ research promises to reshape how we approach Alzheimer’s and potentially improve outcomes for millions affected by this disease.
The study of Alzheimer’s disease encompasses a wide range of inquiries into cognitive decline and memory loss associated with aging. Researchers are increasingly focusing on the intricate interactions within the brain’s immune system, specifically examining the role of glial cells and their influence on neurodegenerative conditions. Exploring the connection between these immune cells and illnesses such as Alzheimer’s offers hope for developing effective therapies. With the aging population and rising cases of dementia-related disorders, the implications of such research are profound, potentially leading to early detection methods and novel treatment strategies. In a quest for breakthroughs, understanding the brain’s immune response remains a pivotal element in combating these challenging diseases.
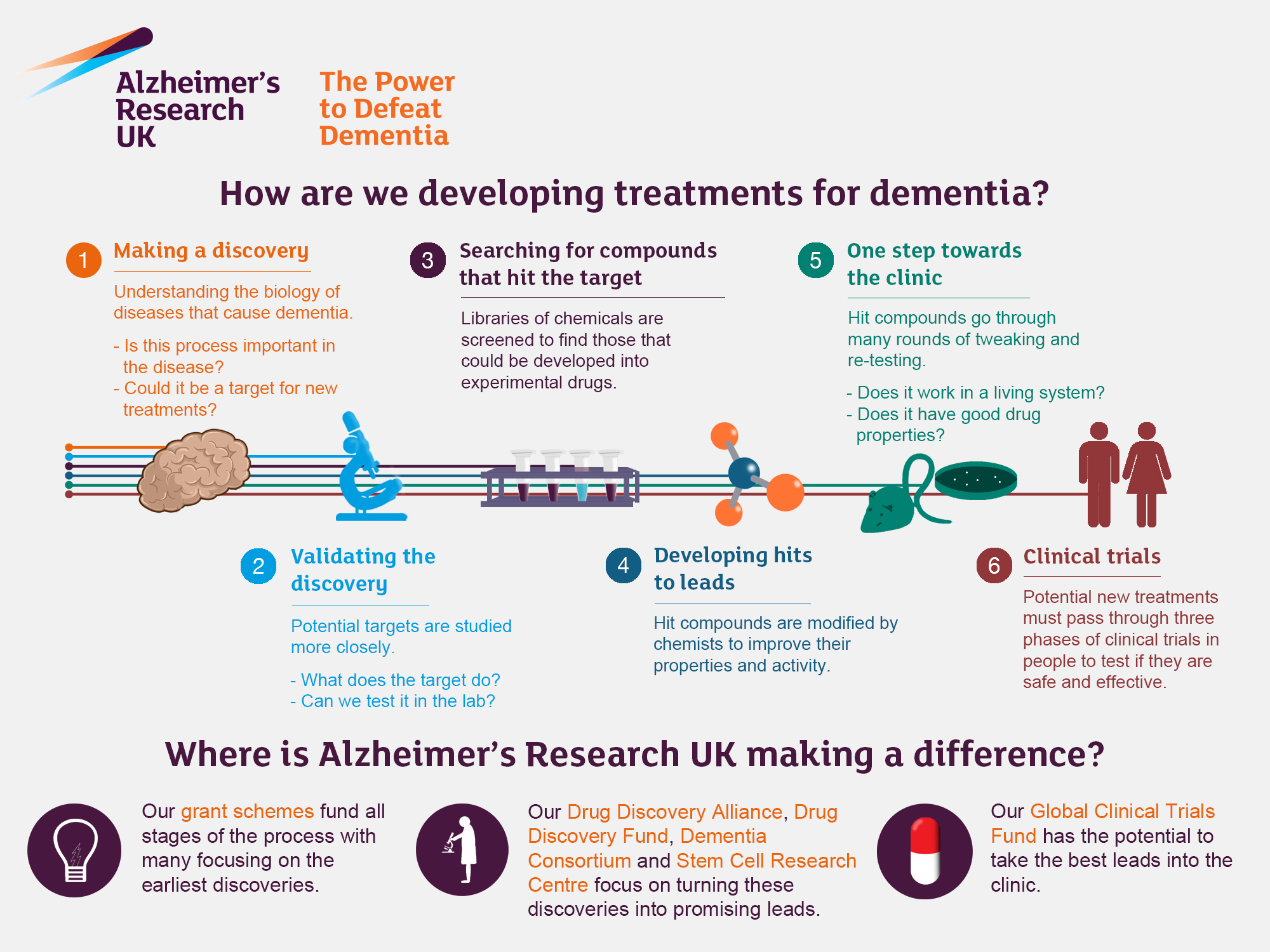
The Role of Microglial Cells in Alzheimer’s Disease
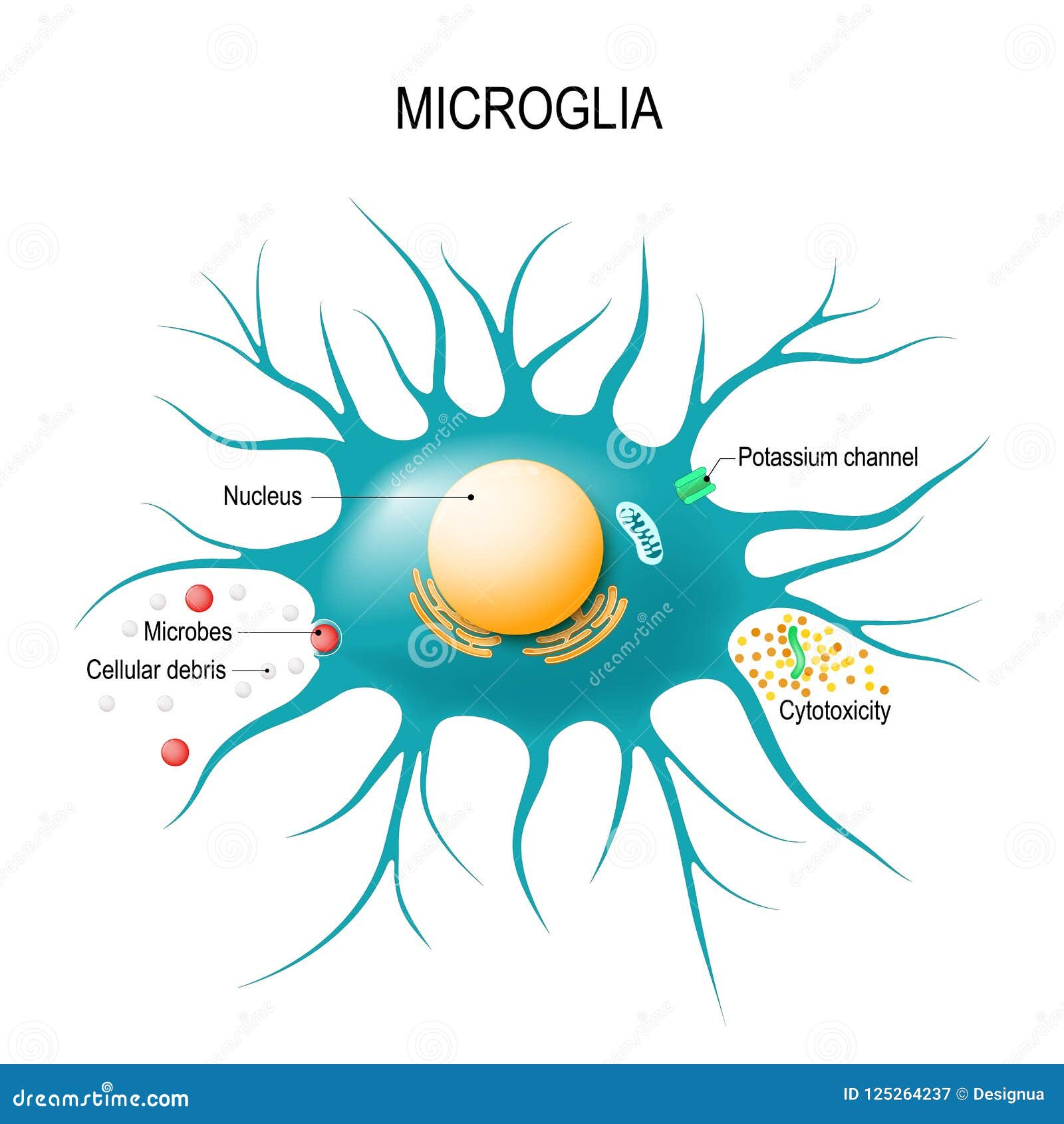
Microglial cells are a critical component of the brain’s immune system, playing a vital role in maintaining neuronal health and homeostasis. Their primary function includes monitoring the brain for injury or signs of illness and responding accordingly by clearing dead or damaged cells. However, in conditions such as Alzheimer’s disease, the functioning of these cells can become compromised. Research led by Beth Stevens has revealed that these cells can mismanage the pruning of synapses, which are crucial for neuronal communication, thereby contributing to the progression of neurodegenerative diseases.
The dysfunction of microglial cells in Alzheimer’s disease highlights the need for further investigations into their role within the brain’s immune response. By understanding how these cells operate, researchers can identify potential therapeutic targets. New strategies aimed at enhancing microglial function might pave the way for innovative Alzheimer’s treatments, ultimately aiming to restore the balance between synaptic pruning and neuronal health.
Beth Stevens: Pioneer in Alzheimer’s Research
Beth Stevens has emerged as a leading figure in the realm of Alzheimer’s research, particularly through her work on microglial cells. With significant contributions from various federal agencies, including the National Institutes of Health, her pioneering studies over the last two decades have shifted the scientific community’s understanding of the brain’s immune system. Stevens acknowledges the importance of curiosity-driven science, which has allowed her to explore uncharted territories in neurodegenerative diseases and their underlying mechanisms.
Stevens’ research serves as a reminder that foundational studies can lead to groundbreaking discoveries. By exploring the visual systems of mice, for instance, she has been able to develop insights that translate into wider implications for human health. The potential to translate her laboratory findings into effective Alzheimer’s treatments underscores her commitment to improving the lives of millions affected by this devastating disease.
The Future of Alzheimer’s Treatments
With the projected increase in the number of Alzheimer’s disease cases expected to double by 2050, the urgency for effective treatments has never been greater. Stevens’ laboratory is paving the way for new medications aimed at combating the underlying issues associated with neurodegenerative diseases. These advances not only strive towards treating Alzheimer’s or Huntington’s disease but also focus on identifying biomarkers that allow for earlier detection of these conditions.
The innovation in Alzheimer’s treatments is closely linked to ongoing research on microglial cells and their implications in disease progression. By targeting the dysfunction in synaptic pruning and enhancing the brain’s immune functions, researchers hope to develop more precise therapeutic strategies, resulting in better outcomes for patients and potentially alleviating some of the economic burdens associated with care costs.
Understanding Neurodegenerative Diseases: A Broader Perspective
Neurodegenerative diseases encompass a variety of conditions, including Alzheimer’s, Huntington’s, and others, all characterized by the progressive degeneration of the nervous system. The interest in understanding how microglial cells can exacerbate or mitigate these diseases opens new avenues for research and treatment. Stevens’ work emphasizes the critical need for a comprehensive understanding of these pathways in potentially altering the course of neurodegeneration.
Moreover, the insights gained from studying neurodegenerative diseases could catalyze developments in related fields of neuroscience. As more researchers delve into the interactions between microglial cells and other neural components, the potential for discovering new treatment options increases, one that might not only extend life but improve the quality of life for those afflicted with neurodegenerative ailments.
The Importance of Biomarkers in Alzheimer’s Detection
Biomarkers play a crucial role in the early detection and diagnosis of Alzheimer’s disease. Stevens and her team are at the forefront of this initiative, working to identify specific biological markers that signal the onset of Alzheimer’s before clinical symptoms manifest. This proactive approach could change the landscape of treatment, allowing for interventions during the early stages of the disease when they might be most effective.
Investigating biomarkers also complements the ongoing research on microglial functionality and the brain’s immune response. By establishing a link between biological indicators and disease progression, research led by Stevens paves the way for creating targeted therapies aimed at modulating these immune responses, significantly enhancing the overall effectiveness of Alzheimer’s treatments.
Impact of Aging on Neurodegenerative Diseases
As the U.S. population ages, the incidence of neurodegenerative diseases such as Alzheimer’s is expected to grow exponentially, highlighting a demographic challenge that influences healthcare systems. Understanding how the aging process affects microglial cell function is essential in developing therapies that address the needs of an older population. Research in this area could lead to tailored treatments that account for the unique biological changes that occur with age.
Incorporating knowledge about the interplay between aging and the immune system of the brain could help scientists design strategic interventions. This research focus could also foster collaborative efforts among neuroscientists, geriatric specialists, and public health officials to create holistic approaches to prevention and treatment, ultimately standing to benefit millions who are at risk for Alzheimer’s disease and other neurodegenerative disorders.
Innovative Approaches to Combating Alzheimer’s Disease
The quest for innovative approaches to combat Alzheimer’s disease encompasses a range of strategies, from drug development to lifestyle interventions. The Stevens Lab emphasizes not only advanced pharmacological treatments but also the importance of understanding the underlying biology of neurodegenerative diseases through basic research. This dual approach serves as a foundation upon which future therapies can be built.
Collaboration across various scientific disciplines is crucial as researchers work together to combine insights from biochemistry, genetics, and immunology. As progress continues in unraveling the complexities of diseases like Alzheimer’s, the application of emerging technologies and novel treatment strategies is likely to offer hope and improved outcomes for individuals affected by these life-altering conditions.
Navigating Challenges in Alzheimer’s Research
Researching Alzheimer’s poses many challenges, primarily due to the complexity of the disease itself. It involves multiple biological systems, environmental factors, and genetic components that contribute to its manifestation. Beth Stevens, through her insightful focus on microglial functions, highlights the necessity in overcoming these hurdles for the progression of effective Alzheimer’s treatments.
Furthermore, securing consistent funding and maintaining public interest in Alzheimer’s research can be significant hurdles. As more data emerges showing the potential impact of research discoveries, it becomes essential for continued support from governmental and private entities to drive forward the fight against Alzheimer’s and related neurodegenerative diseases.
The Role of Federal Funding in Alzheimer’s Research
Federal funding plays a pivotal role in facilitating Alzheimer’s research and development of new treatments. Without the financial support from institutions such as the National Institutes of Health, groundbreaking studies that delve into the mechanisms of diseases, like those conducted by Stevens, may not come to fruition. The emphasis on robust funding is particularly crucial as the prevalence of Alzheimer’s escalates with the aging population.
Moreover, stable funding streams enable researchers to undertake long-term projects that are essential for understanding complex neurodegenerative diseases. Investment in this area not only fosters scientific breakthroughs but also contributes to establishing a comprehensive healthcare strategy that aims to enhance the lives of millions living with Alzheimer’s.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial components of the brain’s immune system and play an essential role in Alzheimer’s research. These cells monitor the brain for damage and help eliminate dead or dysfunctional cells through a process known as pruning. Research from Beth Stevens has shown that malfunctioning microglial activity can contribute to neurodegenerative diseases, including Alzheimer’s, by failing to appropriately prune synapses, which may eventually lead to cognitive decline.
How are Beth Stevens’ findings influencing Alzheimer’s treatments?
Beth Stevens’ groundbreaking research on microglial cells has paved the way for developing new medications aimed at treating Alzheimer’s disease. By understanding how these immune cells can go awry during the disease process, her work offers crucial insights that may lead to innovative treatments and possibly even preventative strategies for neurodegenerative diseases such as Alzheimer’s.
What is the link between neurodegenerative diseases and the brain’s immune system?
Neurodegenerative diseases, including Alzheimer’s, are closely linked to dysfunctions within the brain’s immune system, particularly through the action of microglial cells. Research by Beth Stevens highlights how these immune cells are essential for maintaining brain health, and their improper function can exacerbate neurodegenerative conditions by mismanaging synaptic pruning, resulting in the degeneration of neuronal circuits.
Why is early detection of Alzheimer’s disease important in the context of new research?
Early detection of Alzheimer’s disease is crucial as it allows for timely intervention and potentially better outcomes for patients. Recent studies, including those led by Beth Stevens, have focused on identifying novel biomarkers through microglial activity that can indicate Alzheimer’s earlier in the disease progression, thus enhancing treatment options and patient quality of life.
What funding sources support research advancements in neurodegenerative diseases like Alzheimer’s?
Significant funding from federal agencies such as the National Institutes of Health (NIH) has been instrumental in driving research in neurodegenerative diseases like Alzheimer’s. As emphasized by Beth Stevens, this support allows scientists to explore basic science inquiries that lead to significant medical advancements, including new treatment methodologies and a deeper understanding of disease mechanisms.
How do findings in animal models contribute to Alzheimer’s disease research?
Findings from animal models, such as those studied by Beth Stevens, are vital for Alzheimer’s research as they provide insights that are not easily attainable in human studies. Understanding how microglial cells operate in these models helps researchers unravel the complexities of Alzheimer’s disease, leading to meaningful discoveries that can inform therapeutic strategies.
| Key Points |
|---|
| Beth Stevens focuses on microglial cells as the brain’s immune system. |
| Microglia clear damaged cells and prune synapses but can malfunction, contributing to disorders like Alzheimer’s. |
| Stevens’ lab connects microglial malfunction to neurodegenerative diseases, paving the way for new medications and biomarkers. |
| Research funded by federal agencies like NIH allows exploration of complex questions about brain health. |
| The U.S. population aging is projected to double Alzheimer’s cases by 2050, raising care costs significantly. |
| Basic scientific research is crucial for transforming understanding into effective treatments for Alzheimer’s. |
Summary
Alzheimer’s research is vital for understanding and combating this progressive disease that affects millions. Recent discoveries by neuroscientist Beth Stevens emphasize the role of microglial cells in brain health and how their malfunction can lead to Alzheimer’s and other neurodegenerative disorders. By continuing to follow the science and investing in basic research, we can unlock new treatments that may significantly improve the quality of life for those living with Alzheimer’s.