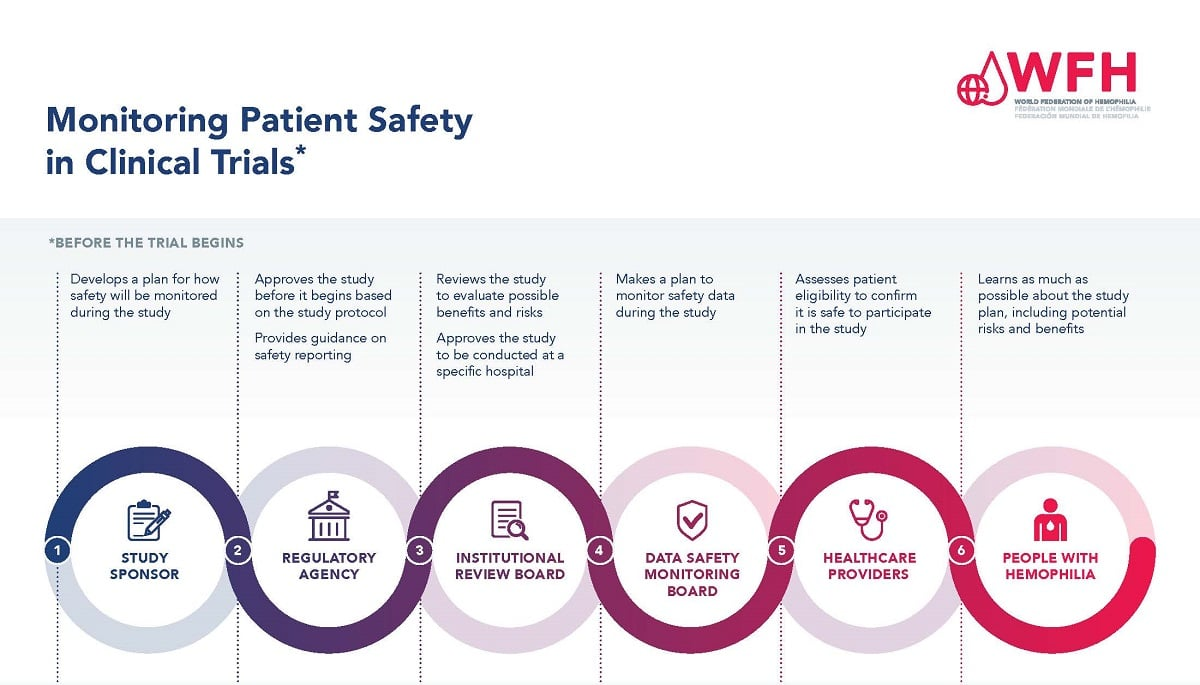
Patient safety in medical research is paramount as it safeguards the well-being of individuals participating in clinical trials. The integrity of these studies relies heavily on robust medical research ethics and stringent oversight by Institutional Review Boards (IRBs), which play a crucial role in protecting research participants. When federal funding, such as NIH grants, is disrupted, it directly impacts the mechanisms ensuring clinical trial safety and research participant protection. The cut in funding not only halts ongoing studies but also breeds mistrust within communities that might hesitate to participate in future research, thus stifling vital medical advancements. As we navigate the complexities of modern research, ensuring patient safety must remain at the forefront of our endeavors.
The protection of individuals involved in clinical studies is a top priority, often referred to through various terminologies such as research participant safeguarding and clinical trial oversight. Effective oversight mechanisms, particularly through the collaboration of IRBs, are designed to uphold ethical standards and reinforce the rights of participants throughout the research process. Disruptions in funding can lead to critical gaps in these protective measures, ultimately risking not only participant safety but also the credibility of the entire research field. As funding streams fluctuate, the commitment to uphold ethical conduct in trials becomes challenged, underscoring the imperative need for continued support and innovation in systems designed to protect those who volunteer for medical research. Understanding these dynamics sheds light on the intricate balance required to foster advancements in medical science while ensuring the safety and rights of those who contribute to it.
The Importance of NIH Funding for Patient Safety in Medical Research
The National Institutes of Health (NIH) plays a crucial role in ensuring patient safety in medical research through its funding programs. NIH funding allows for comprehensive oversight by Institutional Review Boards (IRBs) that are tasked with protecting the well-being of research participants. These IRBs meticulously review research proposals to ensure they meet safety and ethical standards, thus safeguarding participants from potential harm. Without NIH funding, medical research institutions face significant barriers in maintaining these essential oversight mechanisms, potentially compromising patient safety.
Moreover, the financial support from NIH enables seamless collaboration among multiple research sites, fostering innovation and expediting the research process. The introduction of the single IRB (sIRB) model under NIH guidelines streamlines the approval process for multisite studies, ensuring that patient safety measures are uniformly applied across all locations. This model is critical, as it reduces delays and bureaucratic hurdles, thereby allowing researchers to focus on their primary goal—advancing medical knowledge while prioritizing participant welfare.
Frequently Asked Questions
How does patient safety in medical research relate to medical research ethics?
Patient safety in medical research is a fundamental aspect of medical research ethics. It involves ensuring that research participants are protected from harm during studies. Ethical guidelines, such as informed consent and risk assessment, are established to prioritize the welfare of participants, thereby enhancing the integrity and credibility of the research.
What is the role of IRB oversight in ensuring patient safety in medical research?
IRB oversight is crucial for ensuring patient safety in medical research. Institutional Review Boards (IRBs) carefully evaluate research protocols to protect participants from undue risks. They assess study designs, informed consent processes, and monitoring plans to ensure that participants’ rights and well-being are safeguarded throughout the research.
How does NIH funding impact patient safety in medical research?
NIH funding significantly impacts patient safety in medical research by providing financial resources necessary for comprehensive oversight and ethical compliance. These funds support IRB operations and enable institutions to implement robust monitoring and safety measures, ensuring that research participants are protected while advancing scientific knowledge.
What measures are taken to enhance research participant protection in clinical trials?
To enhance research participant protection in clinical trials, measures include thorough IRB reviews, informed consent procedures, ongoing risk assessment, and adverse event monitoring. These practices help mitigate potential risks, ensure participant understanding of the study, and foster trust between researchers and participants.
How can disruptions in funding affect clinical trial safety?
Disruptions in funding can severely compromise clinical trial safety by limiting resources for IRB oversight and participant monitoring. Funding cuts may lead to delayed studies, reduced participant protections, and diminished trust in the research process, ultimately jeopardizing both participant safety and the integrity of the research.
| Key Points | Details |
|---|---|
| Funding Cuts Impact | The halt of over $2 billion in federal research grants disrupts oversight of patient safety in medical research. |
| Role of SMART IRB | SMART IRB manages a system for IRB review that ensures patient protection across multiple research sites. |
| Importance of IRBs | Institutional Review Boards (IRBs) evaluate research proposals to safeguard participant rights and welfare. |
| Historical Context | Past unethical studies highlight the need for rigorous oversight, shaping the current IRB system. |
| Consequences of Funding Cuts | Reduced grants hinder research, potentially causing public mistrust and impacting participant safety. |
Summary
Patient safety in medical research is critically compromised by funding cuts that halt essential oversight mechanisms. The recent freeze on federal research grants limits the ability of Institutional Review Boards (IRBs) to conduct thorough reviews and maintain ethical standards. These cuts can undermine public trust in research, risking the participation of individuals who volunteer for studies that could lead to significant medical advancements. It is imperative that stakeholders recognize the vital role of funding in preserving the integrity of patient safety in medical research.